The dance moves that a water molecule makes as it flips hydrogen bonds from one partner to another have been captured by US researchers. The finding provides compelling evidence to support theoretical predictions of the dynamics of how hydrogen bonds break and reform in aqueous systems.
For many years it has been known that the hydrogen bonds formed by water molecules continuously switch partners - either to other neighbouring water molecules or to dissolved anions. The delay between switching has been calculated, but the mechanism had not been directly observed.
Now, an international team led by Kelly Gaffney at Stanford University in California has shown how the bonds flip extremely rapidly between partners, rather than, for example, as a slower, smoother rotation.
The researchers dissolved sodium perchlorate in water and pulsed the solution with a burst of laser energy, causing the O-H bonds to vibrate at a known frequency, which can be measured. If the H is participating in a hydrogen bond this affects the frequency of the vibration - crucially the vibration frequency varies depending on whether the hydrogen bond is with a neighbouring water molecule or with a perchlorate anion.
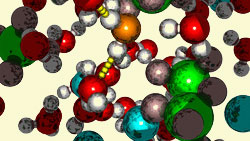
A snapshot from the molecular dynamics simulation showing the hydrogen bonds in aqueous sodium perchlorate
© Michael Odelius, Stockholm University
|
By taking absorption measurements at extremely short intervals, the researchers were able to show that on average the molecule of water remains hydrogen-bonded to a given partner for around 6 picoseconds before it exchanges. And because they could distinguish between one hydrogen bond and another (with either another water as partner or a perchlorate anion), the team could measure how long it took to break one bond and form another. This was only about 50 femtoseconds. Furthermore, by using polarised light and two lasers to interrogate the molecules it was possible to calculate the extent to which a given bond must rotate in order to grasp a new partner. This turned out to be about 50o.
'In other words the molecule makes a hydrogen bond with one partner, then very quickly rotates about 50o to exchange with another partner,' says Gaffney. 'I think we have made an important step forward in complementing theoretical predictions with experimental data that will give us confidence that the simulations are in the right direction.'
Andrew Ellis, an expert on solvation phenomena at the University of Leicester in the UK, rates the work highly. 'This study is a beautiful example of how cutting-edge experiments provide new information on this dynamical process and it specifically shows how hydrogen bond exchange causes the detaching O-H group to swing around, propeller-like, before it reforms a new hydrogen bond with an adjacent molecule.'
Simon Hadlington
Interesting story? Spread the word using the 'tools' menu on the left




