It could be possible to get uranium from seawater in the future, claim US scientists who have devised a new way to extract uranyl ions from aqueous solutions.
With the rapid depletion of fossil fuels, the search for alternative power sources is becoming increasingly important. One alternative is nuclear fission, making uranium - the fuel used in nuclear reactors - an important target for isolation. Although uranium is currently extracted from solid ores such as uraninite, it also exists in large quantities as uranyl ions (UO22+) in seawater. However, due to its distinctive shape that prevents the use of conventional chelating ligands, sequestering the uranyl ion from seawater has remained a challenge.
The uranyl ion binds well with carboxylates, and encasing the ion in an apolar environment stabilises electrostatic interactions and enhances intermolecular forces explains Julius Rebek Jr at Scripps Research Institute in La Jolla. Rebek's team discovered that when three bidentate 2,6-terphenyl carboxylic acid ligands coordinate to the ion, their bulky phenyl groups form a cage around it shielding it from any water. This complex can then be extracted from solution in various ways depending on the type of medium it is in.
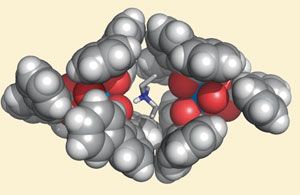
Carboxylic acid ligands coordinate to the ion forming a cage around it
|
'There are about 4.5 billion tons of dissolved uranium in the ocean. This is nearly 1000 times more than the terrestrial uranium sources in the western world,' says Orion Berryman, a member of the research team. 'Our work addresses the challenges of uranium extraction from a unique perspective - isolating the uranium atom from its native environment through encapsulation.'
'It's certainly confronting a significant problem and it's an interesting piece of work,' says Jack Harrowfield, an expert in coordination chemistry at Louis Pasteur University in Strasbourg, France. 'But it needs to be shown that it is selective,' he adds.
Rebek and his colleagues now plan to address this problem by developing ligands that will exhibit a higher affinity for uranium, which will also allow its extraction at much lower concentrations than currently possible.
Yuandi Li
Encapsulation of the uranyl dication
Stephan Beer, Orion B. Berryman, Dariush Ajami and Julius Rebek Jr., Chem. Sci., 2010
DOI: 10.1039/c0sc00116c




