A fuel cell that produces power using only water and a warm breeze has been developed by researchers in Germany. The cell could be used to power sensors and military monitoring devices in remote areas.
Most fuel cells rely on the spontaneous formation of water from the combination of hydrogen and oxygen, with the energy produced determined by the changes in enthalpy between the anode and cathode. Storage of hazardous materials such as hydrogen, methanol or hydrides are needed to run these cells. Now, Emil Roduner and Andreas Dreizler at the University of Stuttgart have developed a concentration fuel cell that runs on water and air, making it cheap, safe and easy to refuel.
In Roduner's system, water is oxidized catalytically to molecular oxygen, protons and electrons at the anode, while the reverse reaction takes place at the cathode. As in normal fuel cells, the cathode and anode are separated by a polymer electrolyte membrane which allows the protons to cross to the cathode while the electrons are forced to make their way through a wire, creating a current. The water that forms at the cathode is evaporated by the air flow, keeping the water concentration gradient between the two electrodes, which acts as the driving force for the reaction.
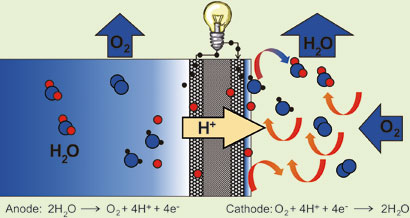
Electrons created at the anode produce current as they travel through a wire to the anode
|
Unlike other fuel cells no change in enthalpy occurs as water reacts to form water. This means that typically minor contributions, such as changes in entropy, become key factors in the energy output, explains Roduner. He adds that his inspiration to create the cell came from a desire to demonstrate that 'changes in entropy can still be a driving force [for fuel cells].'
Michael Janik, an expert on fuel cells at the University of Pennsylvania in Philadelphia, US, agrees that this is an unexpected method to develop a fuel cell. Janik comments that typically '[fuel cell chemists] just look at the fuel and the difference in the fuel versus the activation chemistry but Roduner uses concentration as their driving force - that's clever.'
The energy output is smaller than typical fuels cells but this system may find use in specific situations where a small energy output is needed, such as for powering small sensors or for an emergency signal. Roduner envisions its use in dry windy places, such as along the coast or a desert, to facilitate water evaporation at the cathode.
Patricia Pantos
Enjoy this story? Spread the word using the 'tools' menu on the left or add a comment to the Chemistry World blog.




