Usually, you'd expect two compounds with the same composition, atom-to-atom connectivity and symmetry to be chemically identical too. But scientists investigating metal-organic frameworks have discovered a surprising exception to this rule by identifying two isomers with the same symmetry and bonding but different gas storage properties.
A team led by Shengqian Ma at the Argonne National Laboratory, Illinois, US, investigated a rod-like tetracarboxylate molecule (ebdc) which can bind to a metal atom from any one of four binding points, one at each corner of a rectangle. When it was heated with a copper salt at 75 °C, a crystal phase formed (the  -phase) and at 65 °C a phase with different properties (the
-phase) and at 65 °C a phase with different properties (the  -phase) formed. So far, so normal. But when Ma carried out crystal analysis on these two compounds, he found that they had the same composition, the same atom-to-atom connectivity and the same symmetry. 'This type of symmetry-preserving isomerism has never been observed before in metal-organic frameworks,' says Ma.
-phase) formed. So far, so normal. But when Ma carried out crystal analysis on these two compounds, he found that they had the same composition, the same atom-to-atom connectivity and the same symmetry. 'This type of symmetry-preserving isomerism has never been observed before in metal-organic frameworks,' says Ma.
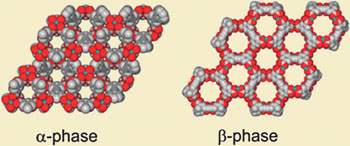
The isomers have the same symmetry but different properties
|
The key to what's happening lies in the ligand, Ma explains. In the  -phase, the ligands bind end-on to each copper unit, whereas in the
-phase, the ligands bind end-on to each copper unit, whereas in the  -phase they bind side-on. This makes the ring larger in the
-phase they bind side-on. This makes the ring larger in the  -phase than in the
-phase than in the  -phase. So, although the same chemical units are joined together with the same sort of bonds and the same overall symmetry, the properties are different. In particular, the area available for hydrogen absorption is significantly higher in the
-phase. So, although the same chemical units are joined together with the same sort of bonds and the same overall symmetry, the properties are different. In particular, the area available for hydrogen absorption is significantly higher in the  -isomer: 'This means that it might be possible to improve gas storage capacities by looking for new isomers of known structures,' says Ma. Paul Forster, a materials chemist at the University of Nevada, Las Vegas, US, is intrigued by what he calls a 'very unusual' form of isomerism. 'Structures that are identical in composition and connectivity, but differ in pore geometry, offer unique and important opportunities to address questions related to the kinetics and thermodynamics of hybrid materials synthesis,' he says.
-isomer: 'This means that it might be possible to improve gas storage capacities by looking for new isomers of known structures,' says Ma. Paul Forster, a materials chemist at the University of Nevada, Las Vegas, US, is intrigued by what he calls a 'very unusual' form of isomerism. 'Structures that are identical in composition and connectivity, but differ in pore geometry, offer unique and important opportunities to address questions related to the kinetics and thermodynamics of hybrid materials synthesis,' he says.
David Barden
Enjoy this story? Spread the word using the 'tools' menu on the left or add a comment to the Chemistry World blog.




