Chemists have uncovered a way to sever two of the strongest bonds in chemistry - in dinitrogen (N2) and carbon monoxide (CO) - and make useful organic compounds. The process works through a hafnium complex - and is an important step towards developing ways to produce important chemicals from abundant gas feedstocks.
Although nitrogen makes up 78 per cent of the atmosphere, it is not used in many industrial processes as the triple bond is difficult to break. The exception is the Haber process to make ammonia - but this requires high pressures and temperatures, as well as hydrogen gas that usually comes from fossil fuel sources.
Alternative chemistry to break the N2 triple bond is highly sought after and could be in great demand in the future. Now, Paul Chirik and colleagues at Cornell University in New York, US, have found a new way to do this - and surprisingly, it works at room temperature.
"Anytime someone finds a new chemical transformation for dinitrogen, we get closer to solving one of chemistry's long standing holy grails"
- Michael Fryzuk, University of British Columbia
'We discovered a method to combine two simple diatomic molecules with very strong bonds: N2 and CO, to make new organic fragments such as oxamide, which is an important slow-release fertiliser,' Chirik told Chemistry World.
Key to the process is a compound called hafnocene, which is a complex of hafnium metal ions with cyclopentadiene and chlorine ligands. The complex can be activated to react with N2 by switching the chlorine for iodine - causing each N2 molecule to be complexed between two hafnocenes. This bonding effectively reduces the triple bond to a single N-N bond.
At this stage, carbon monoxide is added, which breaks the final N-N bond and forms new C-N bonds. By varying the amount of CO added, different organic compounds can be made.
'The downside is that the process is stoichiometric, rather than catalytic - meaning that one hafnium compound is required for every N2 molecule that is cleaved,' Chirik says. 'But this does not deter us from moving forward. We have uncovered a new reaction space and a unique transformation that we hope to apply to catalytic reactions and important industrial processes.'
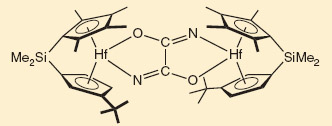
The hafnocene complex allows the CO to be inserted into the strong N-N bond
© Nature Chemistry
|
An unexpected result of the research is the reactivity of CO - which bonds with the held N2 rather than the metal ion, as is commonly expected. Alessandra Quadrelli at the University of Lyon, France, said: 'We are ourselves trying to develop N-C bond formation from N2 - but we did not fathom that stable CO could be the synthon of choice'.
'N-C bond formation from inert, stable and abundant molecules is a dream reaction - since so many valuable compounds in pharmacy and household products have these bonds,' she adds.
Michael Fryzuk, at the University of British Columbia in Canada, agrees that there is great potential in the process. 'This work represents a whole new kind of reactivity for coordinated dinitrogen,' he says. 'Anytime someone finds a new chemical transformation for dinitrogen, we get closer to solving one of chemistry's long standing holy grails: that of utilisation of N2 as a feedstock for producing organonitrogen compounds.'
Lewis Brindley
Interesting? Spread the word using the 'tools' menu on the left.




