Chinese scientists have synthesised a giant cluster containing 60 lanthanum and 76 nickel atoms, the largest of its type.
The four-layer cluster contains both first- and third-row transition metals, making it a member of the so-called 3d-4f family. A variety of these complexes have been made, some with over 100 metal atoms, but this one, which has a maximum dimension of 31 Ångstroms, is the largest so far, says Zhiping Zheng from Xiamen University and colleagues.
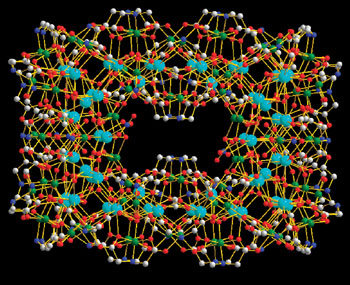
The giant cluster may have an application in molecule-based magnets
|
Zheng's team made the cluster by mixing lanthanum nitrate, nickel nitrate and iminodiacetic acid (a ligand for the reaction) with sodium hydroxide at 80 °C. They filtered the resulting suspension and evaporated the filtrate to give the cluster as blue block-shaped crystals.
Zheng says that determining the compound's structure by single-crystal X-ray diffraction is challenging because of disorder within the crystals. Nevertheless, they were able to deduce the exact arrangement of atoms, and found that the cluster consisted of four distinct shells. In addition, interactions between the metal atoms gives the cluster magnetic properties, something that will be the subject of further work, says Zheng.
The conditions used to make the cluster are better controlled than in syntheses of similar compounds because they avoid the use of high pressure, says Zheng, who adds that 'other closely related clusters may be readily accessible simply by using different transition and lanthanide metal salts.' This, he says, will help to establish the structure-property relationships of these materials, which may have applications as molecule-based magnets.
Lawrence Dahl, an expert in cluster chemistry at the University of Wisconsin Madison, US, is impressed by the work. 'The formation under carefully controlled reaction conditions of this highly organised four-shell architecture is indeed amazing.' He looks forward to further results in the field, saying that Zheng and coworkers have 'opened the door to an exciting new diversity of nanosized transition-metal materials.'
David Barden
Enjoy this story? Spread the word using the 'tools' menu on the left or add a comment to the Chemistry World blog




