In a landmark for silicon chemistry, US researchers have reported the first stable silicon (0) compound to contain a silicon-silicon double bond. The unusual molecule could kickstart the development of new reaction strategies using highly reactive silicon compounds, the researchers suggest.
The disilicon molecule (Si=Si) is far too reactive to exist in isolation. Disilenes (R2Si=SiR2) and disilynes do exist, but the cores in these compounds are no longer in the elemental, highly active, zero oxidation state; their silicon atoms have lost their lone pairs by combining with other molecular fragments.
But Yuzhong Wang and colleagues at the University of Georgia have managed to synthesise a dark red crystal complex whose core effectively has the properties of a diatomic silicon unit. The researchers stabilise the electron-deficient core with a bulky carbene - a coordinating ligand that the group has previously used to isolate diborene.
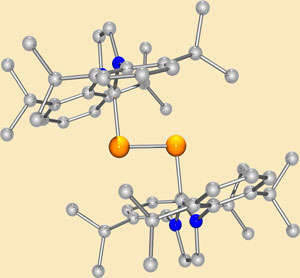
'The diatomic core of our complex can be considered to be a silicon allotrope with two distinct reactive handles: a lone pair of electrons and a double bond.' says principal investigator Gregory Robinson. 'We predict that in combination, these two factors will result in this compound being highly reactive.'
'What baffles me is that this complex is a truly stable, "bottleable" species, not just a molecule which is observed in a low-temperature matrix,' comments Gernot Frenking, professor of theoretical chemistry at the Philipps-Universität in Marburg, Germany.
Guy Bertrand, of the University of California, Riverside, says such molecules, and other zero-oxidation state clusters of main-group elements such as boron and phosphorus, are not just academic curiosities. 'One of the obvious advantages of base-stabillised element(0) compounds is their greater solubility, which facilitates further chemical transformation,' he says.
The intermediate could lead to new ring, polymer and organometallic systems with potentially exciting properties. But the researchers stress that the research is in its infancy and many more studies are required to assess the reactivity and potential applications of the new silicon species.
Fred Campbell
Enjoy this story? Spread the word using the 'tools' menu on the left.




