Scientists in Germany have developed a promising new catalyst that splits water using sunlight -and stores the hydrogen and oxygen produced. The research combines two important energy sources of the future: solar power and hydrogen fuel.
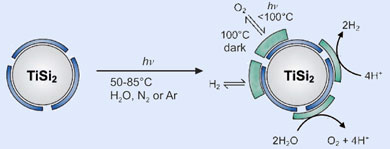
The team at the Max Planck Institute for Bioinorganic Chemistry in Mülheim found that titanium disilicide (TiSi2) could split water using a photocatalytic process akin to photosynthesis - where chlorophyll molecules use light energy to convert water and carbon dioxide into glucose at room temperature. The semiconductor was also able to separate and store the hydrogen and oxygen released - overcoming a problem with earlier methods which released a highly flammable mix of the two gases.
The secret to how this catalyst works lies in the thin layers of titanium oxide (TiO2) and silicon oxide (SiO2) that form on the surface of the TiSi2. These layers protect the catalyst from further corrosion but also give rise to catalytically active centres that enable the reaction to take place.
The team also found that the oxide layers offered a convenient solution to the separation problem. As the reaction occurs, hydrogen and oxygen are absorbed onto the surface of the catalyst and held there. Although storage space is limited, the two gases can be released in different ways - hydrogen is released when the catalyst is cooled to ambient temperature, but oxygen is only released when the catalyst is heated to 100°C in the dark.
Titanium disilicide splits water
© Martin Demuth
|
Previous work in this field has been challenging, says Martin Demuth, who worked on the project.
'Semiconductors suitable for use as photocatalysts have been difficult to obtain, have unfavorable light-absorption characteristics, or decompose during the reaction,' he explained. However, TiSi2 absorbs light across a broad spectrum and is also cheap and readily available.
Demuth and colleagues have founded a new company with the aim of further developing and implementing this technology.
Commenting on the work, Bartek Glowacki, an expert in photocatalysis at the University of Cambridge, told Chemistry World: 'This research certainly shows progress but we are not there yet. For efficient large-scale production of H2 and possibly O2 a different combination of elements may be required, or the use of large surfaces at elevated temperatures.'
Lewis Brindley




